
BESAFE is at the forefront of the fight against substandard and falsified (SF) drugs. Through rigorous research, public health strategies and communications campaigns, we strive to equip healthcare providers, policymakers, manufacturers and consumers with the knowledge and tools required to safeguard their communities from the harms of SF drugs. This group is led by a team of public health practitioners at the Department of Population, Family and Reproductive Health at the Johns Hopkins Bloomberg School of Public Health.
Read the report for the 2024 Symposium on Public Health Strategies for Combating Substandard and Falsified Drugs
Held at the Johns Hopkins University Bloomberg Center in Washington, DC and hosted by the Johns Hopkins Bloomberg School of Public Health, the event brought together more than 100 global leaders, policymakers, and experts to address the critical issue of substandard and falsified drugs affecting public health worldwide.

Our work
BESAFE advances evidence-based strategies to prevent exposure to substandard and falsified medical products by generating actionable research, building strategic cross-sector partnerships, and driving targeted awareness and education initiatives that strengthen trust in health systems and improve patient safety.

Research and Knowledge Generation

Partnerships and Systems Engagement

Awareness, Education, and Action
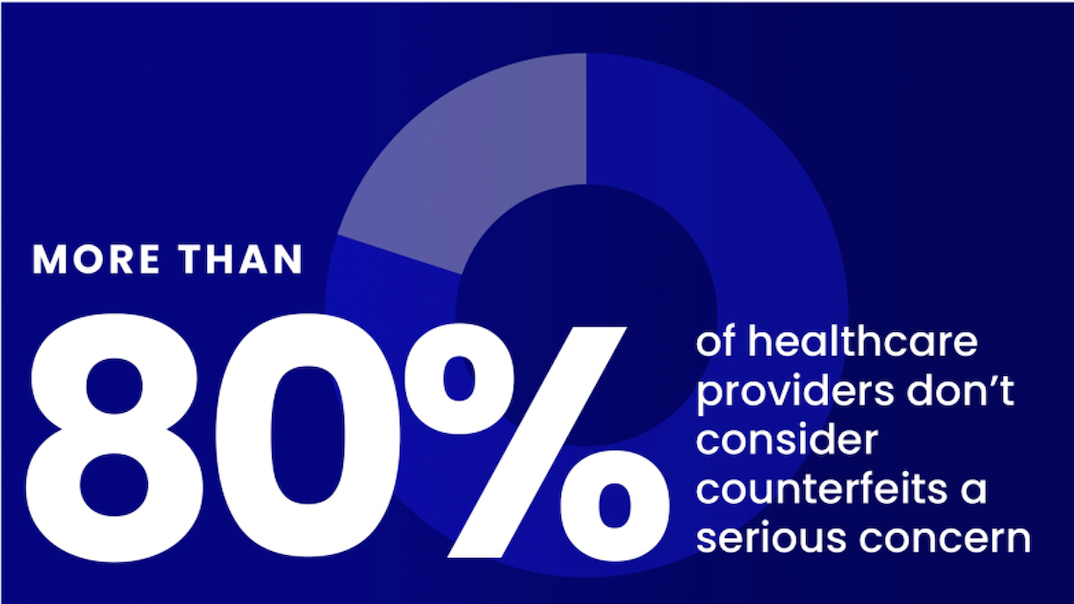
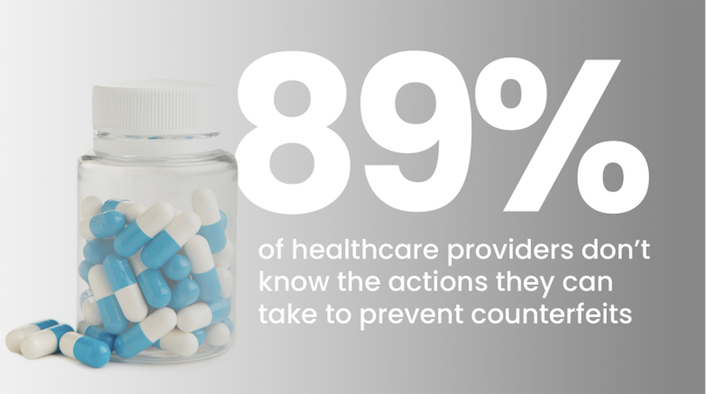
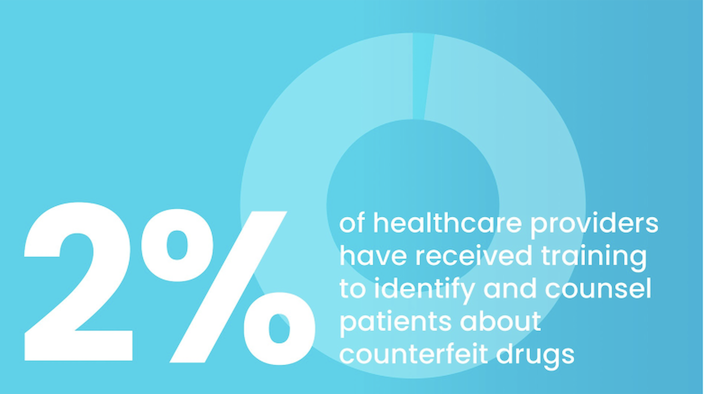
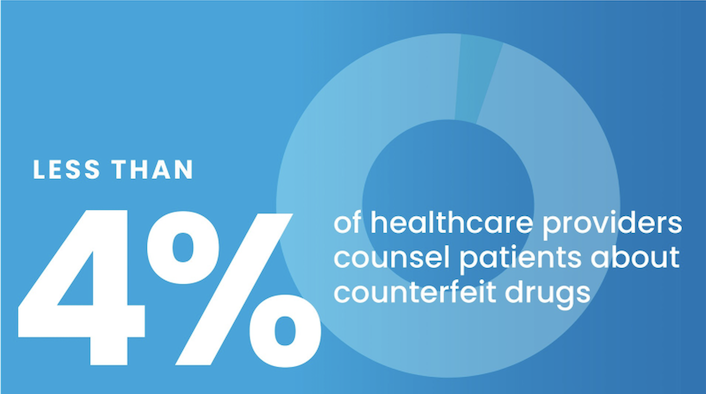
the facts
Substandard and falsified drugs threaten health worldwide
10% of drugs in the market are likely substandard or falsified, with the majority purchased from online pharmacies (WHO). While a dire problem in lower and middle income countries, substandard and falsified drug use is becoming a growing problem high income regions, including in the United States.
SF drugs are found in every continent and almost every country. According to the 2020 Organization for Economic Co-operation and Development (OECD) report, the primary countries from which SF drugs originate are likely China, Hong Kong, Singapore, and India. Read More
Voices for quality: Jean Christophe Rusatira
Voices for Quality features perspectives from individuals on the frontlines of the substandard and falsified medicine crisis — and their…
Globalization of substandard and falsified medicines through internet pharmacies
Globalization of substandard and falsified drugs through internet pharmacies and the risk of purchasing drugs from illegal online pharmacies Industry experts…
Public health responses to substandard and falsified medicines
Although the adverse health impact of substandard and falsified (SF) medicines is catastrophic, it is difficult to measure the overall…
Substandard and falsified medicines are pervasive health threats to the
Substandard and falsified (SF) drugs adversely affect health in multiple ways. These drugs are ineffective due to insufficient or no…
No country is immune from the threat of substandard and
No country is immune; substandard and falsified (SF) drugs were found in every continent and almost every country, from Australia…
Significance
Note that at the Seventieth World Health Assembly in 2017, the WHO adopted the term “Substandard and Falsified (SF) medical…
Resources for Consumers and Practitioners
Up to two billion people around the world lack access to necessary medicines, vaccines, medical devices including in vitro diagnostics, and other health products, which creates a vacuum that is too often filled by substandard and falsified products. This problem is growing as global supply chains become more complex, meaning products manufactured in one country may be packaged in a second country and distributed across borders to be marketed or sold to consumers in a third. More resources here
News and Updates
Monthly BESAFE Newsletter
For academics, industry professionals, practitioners and anyone with an interest in safeguarding public health by making medicines safer. The monthly BESAFE newsletter curates the latest research, news, and opportunities from our research group, and beyond.


















